UPNEEQ
Imagine an eye-opening lift with a daily drop of Upneeq!
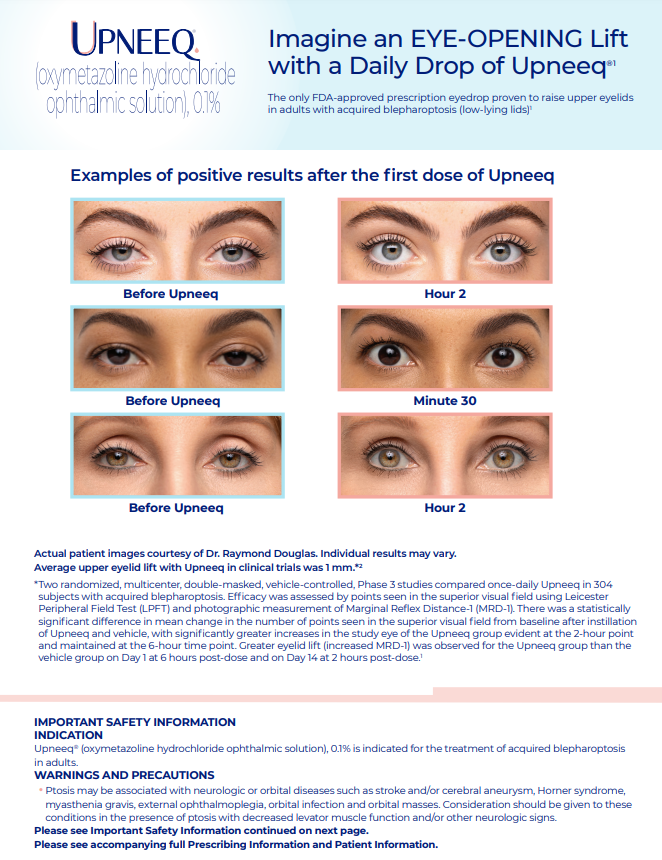
Upneeq is the only FDA-approved prescription eyedrop for acquired ptosis (low-lying lids) that lifts your upper eyelids to open your eyes.
Upneeq is a first-in-class treatment—with a mechanism of action that lifts upper eyelids. The first FDA-approved once-daily prescription eyedrop proven to rapidly lift the upper eyelid. Low-lying lids can affect the way you look and see things.
Directly activates α-adrenergic receptors in Müller’s muscle, stimulating contraction and elevation of the upper eyelids in adults with low-lying lids. Acquired blepharoptosis is a common condition (also called low-lying lids) that can impact adults of all ages. Upneeq stimulates Müller’s muscle to raise the upper eyelid and delivers rapid, visible lift to the upper eyelid with once-daily use. Upneeq significantly improves upper field of vision. In clinical trials, Upneeq helped patients with acquired ptosis see more—on the first day of treatment!
87.8% of patients had some form of improvement.
40.8% of patients had at least a 50% improvement on Day 14 (2 hours after applying Upneeq).
If your eyes look “tired” or “sleepy,” you may have a condition called acquired blepharoptosis (also known as acquired ptosis or low-lying lids). Lifts eyelid(s) quickly. Most patients in clinical trials had a lift in their eyelids in as little as 2 hours. In one study, some patients saw a lift in their eyelids as fast as 5 minutes after the first dose.
84% of patients had some form of improvement.
74% of patients had more than a 50% improvement. Upneeq is also safe and well-tolerated.
In clinical trials, Upneeq was proven to be safe and effective when used as directed.
Common side effects (seen in 1-5% of patients) included eye inflammation, eye redness, dry eye, blurred vision, instillation site pain, eye irritation, and headache
What warnings and precautions are associated with Upneeq?
Upneeq is a type of medication that may affect your blood pressure. If you have heart disease, uncontrolled high or low blood pressure, or feel faint at rest or when quickly standing up, you should call your doctor if your symptoms get worse.
Patients with reduced blood flow to the brain or heart, or patients who experience eye or mouth dryness due to an immune system disorder (Sjögren’s syndrome), should use care when taking Upneeq. Call your doctor immediately if you feel your symptoms may be getting worse.
Upneeq may increase the risk of eye pressure due to fluid buildup (angle-closure glaucoma) in patients with untreated narrow-angle glaucoma. Call your doctor immediately if you feel increased pressure in your eye after using Upneeq.
Do not let the tip of the Upneeq vial touch your eye or any other surface. This can help prevent eye injury or contamination. Each Upneeq vial is for one-time use and should be discarded after being used.